
Essential Tips for Sourcing Pharmaceutical Intermediates?
Sourcing Pharmaceutical Intermediates can be a complex task. Many companies face significant challenges during this process. The right intermediates are vital for producing quality drugs. Missteps can lead to increased costs and delays.
In today’s global market, finding reliable suppliers is crucial. Communication is key. It's not just about cost; quality matters deeply. Companies must perform due diligence. Research potential suppliers, their reputations, and production capabilities. These steps can prevent future mishaps.
A well-thought-out sourcing strategy can reduce risks. Consider factors like supply chain stability. Also, think about regulatory compliance. Remember, sourcing isn’t simply transactional. Building strong relationships is essential. A partnership with good intermediates can boost long-term success.

Understanding Pharmaceutical Intermediates: Definition and Importance
Pharmaceutical intermediates play a critical role in drug manufacturing. They serve as building blocks in the synthesis of active pharmaceutical ingredients (APIs). Understanding their importance helps in ensuring the efficacy and safety of medications. According to a recent industry report, the global pharmaceutical intermediates market is expected to reach $25 billion by 2025. This growth emphasizes the vital need for a reliable supply chain.
Sourcing these intermediates can be challenging. Quality control is a significant concern. Reports indicate that over 30% of intermediates fail to meet quality standards, leading to delays. Trust and transparency in supplier relationships are essential. Businesses must vet their suppliers meticulously. This includes assessing manufacturing capabilities and compliance with regulatory standards.
Awareness of fluctuating prices is also important. Market conditions can lead to sudden cost increases. Companies often overlook the long-term implications of sourcing decisions. The risk of supply disruptions can jeopardize production timelines. Smart sourcing strategies must account for these factors to avoid pitfalls. The path may not always be straightforward, but vigilance and informed choices can make a difference.
Essential Tips for Sourcing Pharmaceutical Intermediates
This chart illustrates the importance of various factors when sourcing pharmaceutical intermediates. Quality and supplier reputation are the most significant, influencing overall decisions in the pharmaceutical supply chain.
Key Considerations for Selecting Reliable Suppliers of Intermediates
When selecting suppliers for pharmaceutical intermediates, several key considerations come into play. It's essential to assess their quality assurance processes. Look for suppliers with robust certifications. This often indicates a commitment to maintaining high standards. Ask for documentation that demonstrates their adherence to industry regulations. A certified supplier frequently produces safer and more reliable intermediates.
Pricing is another important factor, but don’t let it be the only driver for your decision. A lower price can sometimes indicate subpar quality. It's crucial to balance cost with quality. Engage in conversations to understand their production capabilities. Insight into their processes can reveal much about their reliability. Don’t hesitate to ask for references or case studies. A solid supplier is usually proud to share success stories.
Also, consider the supplier's communication style. Responsive, transparent communication is vital for successful partnerships. You want a partner who will be available to address concerns promptly. If a supplier delays responses, it might reflect their overall reliability. Pay attention to these subtle signals. They often reveal more than what is on the surface. Trust your intuition when evaluating potential suppliers.
Essential Tips for Sourcing Pharmaceutical Intermediates
| Consideration | Description | Importance Level | Potential Risks |
|---|---|---|---|
| Supplier Reputation | Evaluate the track record and reviews of the suppliers. | High | Low-quality materials or non-compliance with regulations. |
| Quality Assurance Standards | Confirm that the supplier meets industry standards such as GMP. | Critical | Health risks and product recalls. |
| Regulatory Compliance | Ensure suppliers comply with local and international regulations. | High | Legal issues and financial penalties. |
| Technical Capability | Assess the supplier's ability to produce required intermediates. | Medium | Inefficient production and delivery delays. |
| Pricing and Payment Terms | Evaluate pricing structures against budget constraints. | Medium | Over-budget costs and cash flow issues. |
| Communication and Support | Assess the responsiveness and clarity of communication. | High | Misunderstandings leading to errors in orders. |
Evaluating Quality Assurance Standards in Pharmaceutical Sourcing
Evaluating quality assurance standards in pharmaceutical sourcing is crucial for success. Pharmaceutical intermediates are essential for drug development. When sourcing these materials, ensure suppliers adhere to stringent quality standards. Look for certifications that reflect adherence to good manufacturing practices (GMP). These include ISO certifications and local regulations.
Consider the supplier’s auditing process. Regular audits can help maintain high-quality products. Verify that suppliers have a robust quality control mechanism. This includes testing batches for purity and potency. It’s beneficial to develop strong relationships with suppliers to foster open communication about quality issues. Reflections on previous sourcing experiences can provide insights.
Evaluate the documentation process as well. Proper records of quality checks can help in tracing issues. Suppliers must provide certificates of analysis (CoA) for intermediates. This ensures accountability. Analyzing these documents can highlight potential risks. Always question what you might overlook. Being diligent in checking these details can save time and resources in the long run.
Navigating Regulatory Compliance in Pharmaceutical Intermediates
Navigating regulatory compliance in the pharmaceutical industry is crucial. Compliance ensures safety and efficacy, making it an essential step in sourcing pharmaceutical intermediates. In recent years, the market for intermediates has expanded significantly, projected to reach $50 billion by 2027. This rapid growth emphasizes the need for a keen understanding of regulatory frameworks.
Pharmaceutical intermediates must meet stringent regulations set by global entities. For example, the FDA and EMA have specific guidelines that dictate quality and safety standards. Companies often face challenges in maintaining compliance while sourcing these intermediates. A report from the International Pharmaceutical Federation indicates that 40% of manufacturers struggle with regulatory adherence. This indicates a pressing need for robust compliance mechanisms.
Documentation is key in this landscape. Thorough records must be maintained to verify compliance with regulatory requirements. Insight from the World Health Organization indicates that a lack of proper documentation is a common pitfall, leading to costly delays. Creating a culture that prioritizes regulatory training can help mitigate these risks. Staying informed on evolving regulations is not just beneficial; it’s a necessity in today’s dynamic market.
Strategies for Cost-Effective Sourcing of Pharmaceutical Intermediates
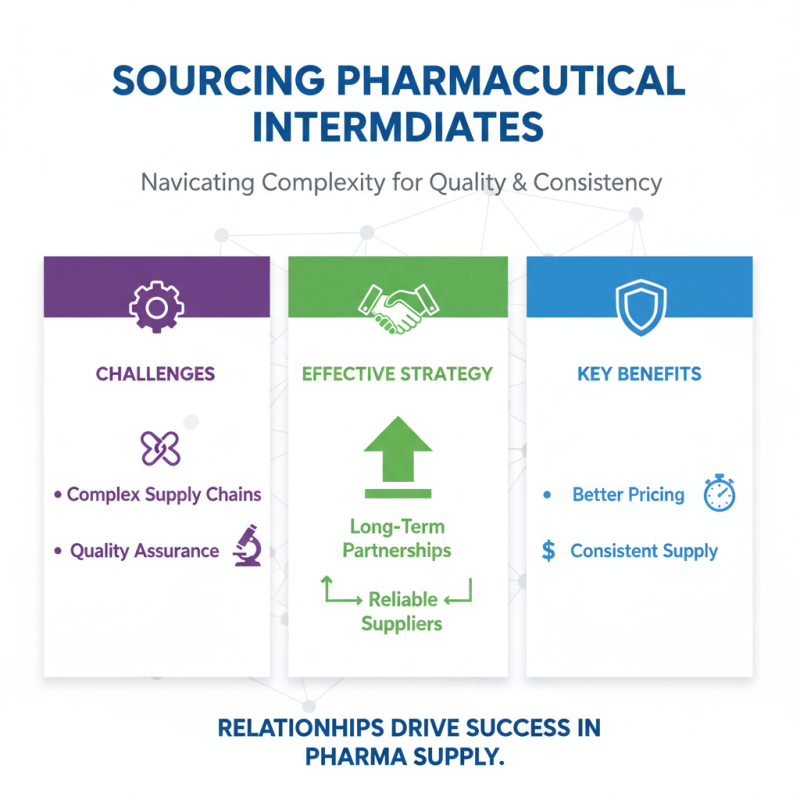
Sourcing pharmaceutical intermediates can be challenging. It often requires navigating complex supply chains and ensuring high quality. One effective strategy is to establish long-term partnerships with reliable suppliers. This can lead to better pricing and consistent supply. Relationships matter in this industry.
Another way to save costs is by evaluating multiple suppliers. Obtaining quotes from various sources can reveal pricing disparities. It’s crucial to assess not just the cost but also the reliability of the supplier. Sometimes, the lowest price comes with hidden risks. Perform thorough due diligence before making commitments.
Consider adopting a phased approach to sourcing. Start with smaller batches to gauge supplier performance. This minimizes financial risk while allowing for quality control. Make sure to track and analyze the delivery times and product quality. Reflection on past sourcing decisions can lead to improved future strategies. Each experience offers valuable lessons.
Related Posts
-

7 Best Strategies for Sourcing High-Quality Pharmaceutical Intermediates in 2023
-

Evaluating the Best Pharmaceutical Chemicals: A Comprehensive Buyer’s Guide
-

Unveiling the Specifications of the Best Raw Pharmaceutical: A Comprehensive Sourcing Guide for Global Buyers
-
5 Essential Tips for Sourcing Quality Pharmaceutical Materials
-

How to Optimize Your Supply Chain for Drug Raw Material Sourcing and Compliance
-

5 Expert Tips to Source High-Quality Pharmaceutical Raw Materials in 2023
