
What is Pharma Material and Its Importance in Drug Development
The significance of Pharma Material in the drug development process cannot be understated, as it forms the foundational basis for formulating effective and safe medications. According to a report by the International Pharmaceutical Federation (FIP), over 70% of the pharmaceutical products rely on high-quality excipients and active pharmaceutical ingredients (APIs) that comprise Pharma Materials. These materials not only affect the stability and bioavailability of drugs but also ensure regulatory compliance, which is crucial for gaining market approval.
Dr. Emilia Ricci, a leading expert in pharmaceutical sciences, emphasizes, "The quality of Pharma Material directly impacts the therapeutic outcome and the overall success of drug development." Her insights highlight that investing in high-quality Pharma Materials is essential for pharmaceutical companies aiming to innovate and bring new therapies to the market efficiently. As the industry continues to evolve with advanced technologies and rigorous standards, the emphasis on high-quality Pharma Materials remains pivotal in ensuring patient safety and therapeutic efficacy. The ongoing integration of cutting-edge research into Pharma Material development is also expected to reshape the landscape of drug formulation and delivery in the coming years.
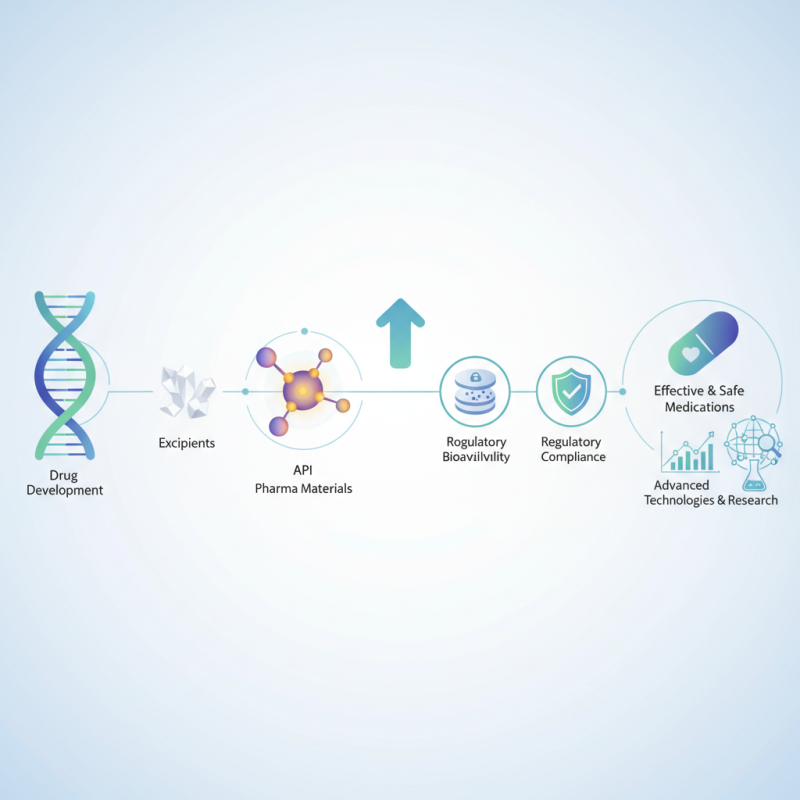
Definition of Pharma Material and Its Components
Pharmaceutical materials, often referred to as pharma materials, encompass a broad range of substances essential in the drug development process. These materials include active pharmaceutical ingredients (APIs), excipients, and packaging components that play critical roles in formulation and efficacy. APIs are the primary ingredients that produce a therapeutic effect in a drug, while excipients serve as inactive substances that aid in the delivery, stability, and absorption of the active ingredients. Together, these components ensure that medications are safe, effective, and manufactured consistently.
The importance of selecting the right pharma materials cannot be overstated, as they directly impact the quality and performance of the drug. The compatibility of the API with excipients, the quality of raw materials, and the integrity of packaging are all crucial factors that influence a drug’s effectiveness and shelf life. Additionally, regulatory compliance requires thorough testing and validation of these materials to ensure they meet safety and efficacy standards. Thus, understanding the components of pharma materials and their interrelations is vital for successful drug development and manufacturing processes.
The Role of Pharma Materials in Drug Formulation
Pharma materials play a crucial role in drug formulation, serving as the building blocks of any medicinal product. These materials encompass a wide range of substances, including active pharmaceutical ingredients (APIs), excipients, and packaging components. The quality and compatibility of these materials directly influence the efficacy, safety, and stability of the final drug product. According to a report by the International Pharmaceutical Federation, roughly 70% of the drug development process involves selecting and optimizing these materials to ensure they meet stringent regulatory standards and patient needs.
In the formulation process, excipients, which are inert substances formulated alongside the active ingredient, serve various functions such as stabilizing, preserving, and enhancing bioavailability. A study conducted by the Pharmaceutical Research and Manufacturers of America highlights that over 90% of the data reported in drug stability studies relate to the interaction between APIs and excipients. This interaction can dictate the release profile and overall therapeutic effectiveness of the medication. Additionally, advancements in materials science are opening new avenues for novel drug delivery systems, which rely heavily on innovative pharma materials to achieve targeted and sustained release mechanisms. Proper selection and formulation of these materials are vital, as they can significantly shorten the development timeline and reduce costs, ultimately leading to more accessible treatments for patients.
Types of Pharma Materials Used in Pharmaceutical Development
In pharmaceutical development, various types of materials play crucial roles in formulating effective and safe medications. Active Pharmaceutical Ingredients (APIs) are among the most significant types of pharma materials. They are the biologically active components that provide the intended therapeutic effect in a drug. The quality and purity of APIs must be rigorously maintained, as even slight variations can impact the drug's efficacy and safety profile.
Excipients are another vital category of pharmaceutical materials. These substances serve multiple purposes, such as aiding in drug absorption, stability, and overall formulation processes. Common excipients include binding agents, fillers, preservatives, and stabilizers, each selected carefully to enhance the drug delivery system. The right combination of excipients can greatly influence the release profiles of the drugs, thus optimizing their performance in the human body.
Additionally, packaging materials are essential in protecting the integrity of pharmaceutical products. They ensure that the medications remain uncontaminated and stable throughout their shelf life. The choice of packaging material can also affect the drug's efficacy and user compliance. High-quality materials that safeguard against moisture, light, and oxygen ingress are critical to maintaining the therapeutic value of pharmaceuticals.
What is Pharma Material and Its Importance in Drug Development
| Type of Pharma Material | Description | Importance in Drug Development |
|---|---|---|
| Active Pharmaceutical Ingredients (APIs) | The substance that produces the intended therapeutic effect. | Critical for the efficacy of the drug; their quality directly impacts the safety and effectiveness of the medication. |
| Excipients | Inert substances used as a carrier for the active ingredients. | They aid in the stability, bioavailability, and patient acceptability of the drug. |
| Biologics | Products derived from living organisms, including proteins, vaccines, and cell therapies. | Important for treating diseases that are not responsive to traditional pharmaceuticals. |
| Packaging Materials | Materials used to contain and protect pharmaceutical products during storage and transport. | Essential for maintaining product integrity and compliance with regulatory standards. |
| Stabilizers | Additives that improve the shelf life and stability of the pharmaceutical formulation. | Reduce degradation of the active ingredient, ensuring efficacy throughout the product's lifespan. |
Impact of Pharma Materials on Drug Efficacy and Safety
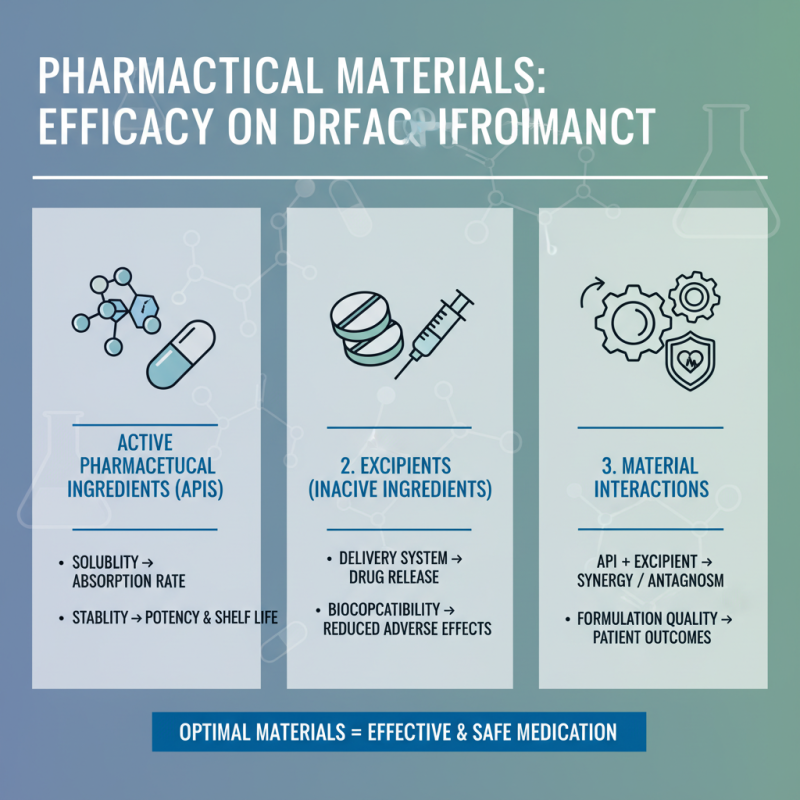
Pharmaceutical materials play a critical role in influencing both the efficacy and safety of drugs. The choice of excipients, active pharmaceutical ingredients (APIs), and their interactions can significantly affect how a drug performs once administered. For instance, the solubility and stability of an API can determine its absorption rate in the body, which directly impacts its therapeutic effectiveness. A well-formulated drug should not only deliver the right dosage but also maintain its potency throughout its shelf life, which relies heavily on the quality and properties of the materials used.
Moreover, the safety profile of a drug is intricately linked to its components. The selection of hypoallergenic excipients can mitigate adverse reactions in sensitive populations, while contaminants in pharmaceutical materials can lead to toxicity or therapeutic failures. Rigorous quality control measures in the sourcing and handling of these materials are essential to avoid impurities that may compromise patient safety. Therefore, understanding the relationship between pharma materials and drug performance is essential for developing safe, effective medications that meet the needs of diverse patient populations.
Regulatory Considerations for Pharma Materials in Drug Manufacturing
In drug manufacturing, regulatory considerations for pharma materials are critical to ensure safety, efficacy, and compliance with industry standards. Regulatory agencies, such as the FDA and EMA, set strict guidelines for the sourcing, testing, and usage of active pharmaceutical ingredients (APIs) and excipients. These materials must undergo rigorous validation processes to confirm their quality and consistency, as any variability can significantly impact the final product's performance.
Tips: Always maintain thorough documentation of all materials used in formulation processes. This practice not only supports compliance with regulatory requirements but also enhances traceability and facilitates auditing processes.
Additionally, companies must be aware of the Good Manufacturing Practice (GMP) regulations that govern how pharma materials are handled throughout production. This includes ensuring that raw materials are sourced from accredited suppliers and that there are extensive protocols for storage and handling to minimize contamination risks. Non-compliance can lead to severe repercussions, including product recalls and legal actions.
Tips: Regularly conduct internal audits and training sessions for staff to keep them updated on regulatory changes and best practices in handling pharma materials. This proactive approach helps mitigate risks and reinforces a culture of quality within the organization.
Importance of Pharma Materials in Drug Development
Related Posts
-

Navigating the Future of Best Pharmaceutical Materials in 2025 and How to Stay Ahead
-

7 Best Strategies for Sourcing High-Quality Pharmaceutical Intermediates in 2023
-

Unique Uses of Pharmaceutical Chemical Powder in Modern Medicine: Unveiling Real-World Applications
-
5 Essential Tips for Sourcing Quality Pharmaceutical Materials
-

How to Select the Right Pharmaceutical Chemicals for Your Manufacturing Process
-

How to Optimize Your Supply Chain for Drug Raw Material Sourcing and Compliance
