
Top 10 Tips for Efficient Peptide API Manufacturing Process
In the rapidly evolving field of Peptide API Manufacturing, efficiency is paramount in ensuring product quality and meeting market demands. Noted expert Dr. Sarah Thompson, a leading researcher in peptide synthesis, states, "Streamlining the peptide manufacturing process not only enhances productivity but also significantly reduces costs, paving the way for more innovative therapies." As the pharmaceutical industry continues to pivot towards biologics, understanding the intricacies involved in peptide synthesis becomes vital for manufacturers looking to stay competitive.
This article explores the top 10 tips for optimizing the peptide API manufacturing process. By integrating best practices from research and development to production, manufacturers can improve yield and efficiency while maintaining stringent quality standards. From refining purification techniques to embracing automation, every element plays a crucial role in achieving operational excellence in Peptide API Manufacturing. As we delve into these strategies, it’s essential to recognize the impact they can have not just on cost-effectiveness, but also on the overall advancement of peptide-based therapeutics in healthcare.

Understanding the Peptide API Manufacturing Process
The peptide API manufacturing process is a complex and multistep endeavor that requires precision and understanding of biopharmaceutical principles. According to a report by Grand View Research, the global peptide therapeutics market is projected to reach over $50 billion by 2026, indicating a growing demand for efficient manufacturing processes. This escalation necessitates a deeper comprehension of the peptide synthesis and purification methods involved.
In the early stages of the peptide API manufacturing process, solid-phase peptide synthesis (SPPS) is often employed due to its high efficiency and scalability. SPPS allows for the automation of peptide assembly, which can significantly reduce labor costs and production times. Moreover, advancements in purification techniques, including high-performance liquid chromatography (HPLC), are critical for achieving the required purity levels, often over 95% for therapeutic applications. Recent studies have shown that using optimized purification methods can enhance overall yield and quality, directly impacting the cost-effectiveness of production.
Furthermore, the integration of quality-by-design (QbD) principles throughout the manufacturing process is transforming peptide API production. By focusing on the understanding of raw material attributes and process parameters, manufacturers can improve the robustness of their processes and minimize variability. This systematic approach not only aligns with regulatory expectations but also can lead to a reduction in batch failures, which, according to the Pharmaceutical Quality Research Institute, can account for up to 30% of production delays. Embracing these innovative methodologies in peptide API manufacturing is essential for meeting the increasing global demand while maintaining high-quality standards.
Key Factors Affecting Peptide Manufacturing Efficiency
The efficiency of peptide API manufacturing is influenced by several key factors, which are pivotal in optimizing yield and reducing production costs. According to a report by Grand View Research, the global peptide therapeutics market is expected to reach $54.8 billion by 2025, underscoring the need for efficient manufacturing processes. Factors such as reaction optimization, purification techniques, and quality control play significant roles in determining the overall efficiency of production.
To enhance production efficiency, one crucial tip is to invest in advanced synthesis techniques. Utilizing automated synthesizers can significantly reduce labor costs and time, thereby accelerating the production timeline. Recent studies emphasize that implementing parallel synthesis methods can lead to increased throughput and reduction in cycle times by up to 50%.
Another important aspect is the optimization of purification processes. Employing more efficient chromatography methods not only improves yield but also minimizes solvent consumption. A report by MarketsandMarkets highlights that advancements in purification technology can enhance recovery rates by as much as 30%. Thus, focusing on these key manufacturing factors not only boosts efficiency but also ensures high-quality peptide APIs that meet regulatory standards.
Top 10 Tips for Efficient Peptide API Manufacturing Process
Best Practices for Streamlining Peptide Synthesis
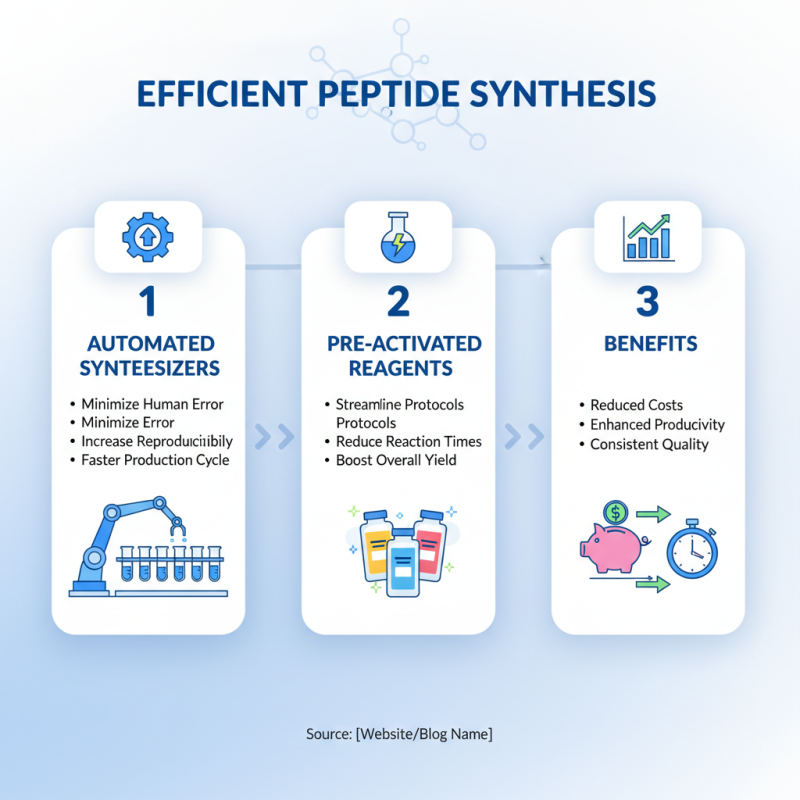
Efficient peptide synthesis is crucial for reducing costs and enhancing productivity in the manufacturing process. One of the best practices for streamlining peptide synthesis is the implementation of automated synthesizers, which greatly minimize human error and increase reproducibility. These machines can perform multiple processes simultaneously, allowing for a more rapid production cycle. Additionally, using pre-activated reagents can further streamline protocols, reducing reaction times and increasing overall yield.
Another critical aspect is the optimization of reaction conditions. Careful selection of reaction solvents and temperatures can significantly impact the speed of synthesis and the purity of the final product. Conducting preliminary experiments to map out these parameters can save time in the long run. Furthermore, integrating real-time monitoring techniques enables manufacturers to make adjustments on the fly, ensuring that any deviations are swiftly corrected. Lastly, fostering a culture of continuous improvement and feedback within the team can lead to innovative solutions that enhance the efficiency of the peptide synthesis process.
Quality Control Measures in Peptide API Production
Quality control in peptide API production is critical to ensuring that the end product meets the necessary safety and efficacy standards. Implementing rigorous quality control measures can significantly impact the overall manufacturing process. One essential aspect is the establishment of standardized testing protocols throughout the production stages. This includes regular analysis of raw materials, intermediates, and the final peptide product to identify any inconsistencies or contaminants early in the process.
To enhance the efficiency of the peptide manufacturing process, incorporating real-time monitoring systems can provide valuable insights into the production environment. These systems allow for immediate detection of deviations from established parameters, enabling prompt corrective actions. Additionally, implementing a robust documentation system ensures that every step of the production and quality control processes is recorded and traceable, which is vital for compliance and audits.
Furthermore, training personnel in Good Manufacturing Practices (GMP) is another critical tip. A well-informed workforce is essential for maintaining quality control throughout the peptide synthesis and purification processes. Regular training sessions can equip staff with the necessary knowledge to identify potential quality issues, fostering a culture of quality within the organization. Adopting these measures can streamline peptide API production while ensuring the highest standards of quality are met.
Top 10 Tips for Efficient Peptide API Manufacturing Process - Quality Control Measures in Peptide API Production
| Tip No. | Quality Control Measure | Description | Frequency | Responsible Party |
|---|---|---|---|---|
| 1 | Raw Material Inspection | Ensure all raw materials meet quality specifications before use. | Before production | QC Team |
| 2 | In-Process Control | Monitor and document critical parameters during processing. | Continuous | Production Team |
| 3 | Final Product Testing | Conduct thorough testing on the final API for purity and potency. | Post-production | QC Team |
| 4 | Equipment Calibration | Ensure all manufacturing equipment is calibrated to maintain accuracy. | Quarterly | Maintenance Team |
| 5 | Documentation Control | Maintain accurate records of all production processes and outcomes. | Ongoing | All Staff |
| 6 | Stability Testing | Assess the stability of the API under various conditions. | Annually | QC Team |
| 7 | Personnel Training | Regular training sessions for staff on quality standards and procedures. | Bi-Annually | HR and QC Team |
| 8 | Supplier Audits | Audit suppliers regularly to ensure they meet quality requirements. | Annually | Supply Chain Team |
| 9 | Change Control | System for managing changes to materials, processes, and equipment. | As needed | QA Team |
| 10 | Complaint Handling | Process for addressing complaints regarding the API as they arise. | Ongoing | Customer Service and QA Team |
Innovative Technologies for Enhancing Peptide Production Efficiency
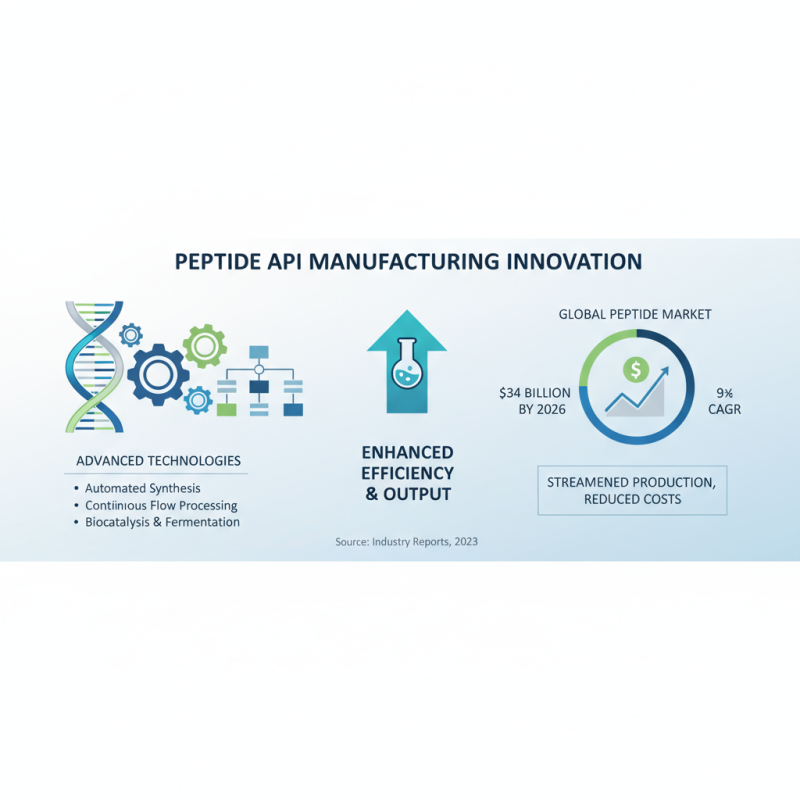
Innovative technologies are rapidly transforming the peptide API manufacturing process, leading to enhanced efficiency and output. Recent industry reports indicate that the global peptide market is projected to reach $34 billion by 2026, with a compound annual growth rate (CAGR) of over 9%. This significant growth underscores the necessity for manufacturers to adopt cutting-edge technologies that streamline production and reduce costs.
One key technology reshaping peptide production is automated synthesizers, which can minimize labor costs and human error while ensuring consistent quality in synthesis. Incorporating process analytical technology (PAT) allows for real-time monitoring of reactions, ensuring optimal conditions are maintained throughout the process. Implementing these technologies can reduce production time by up to 30%, according to an analysis conducted by a leading market research firm.
Another crucial aspect is the integration of continuous flow reactors, which provide a more efficient alternative to traditional batch processing. Continuous flow processing can significantly enhance scalability and reduce solvent usage, leading to a more sustainable manufacturing approach. Manufacturers adopting this method have reported reductions in cycle times by nearly 50%. Emphasizing these innovative solutions, such as automating quality control and utilizing real-time data analysis, will not only improve manufacturing efficiency but also ensure compliance with stringent regulatory standards in the peptide industry.
Related Posts
-

Maximizing ROI: The After-Sales Service Edge in Best Peptide API Manufacturing
-

Ultimate Guide to Sourcing the Best Peptide Api Manufacturing for Your Business Needs
-

China's Resilient Growth in Peptide API Manufacturing Amidst US China Trade Tariff Challenges
-

10 Best Practices for Peptide API Manufacturing to Maximize Efficiency
-

How to Effectively Scale Peptide API Manufacturing for Optimal Yield and Purity
-

Exploring Peptide Api Manufacturing Opportunities at 2025 China 138th Canton Fair with Market Growth Insights
