
The Best Drug Raw Material Sources for Quality Pharmaceutical Production?
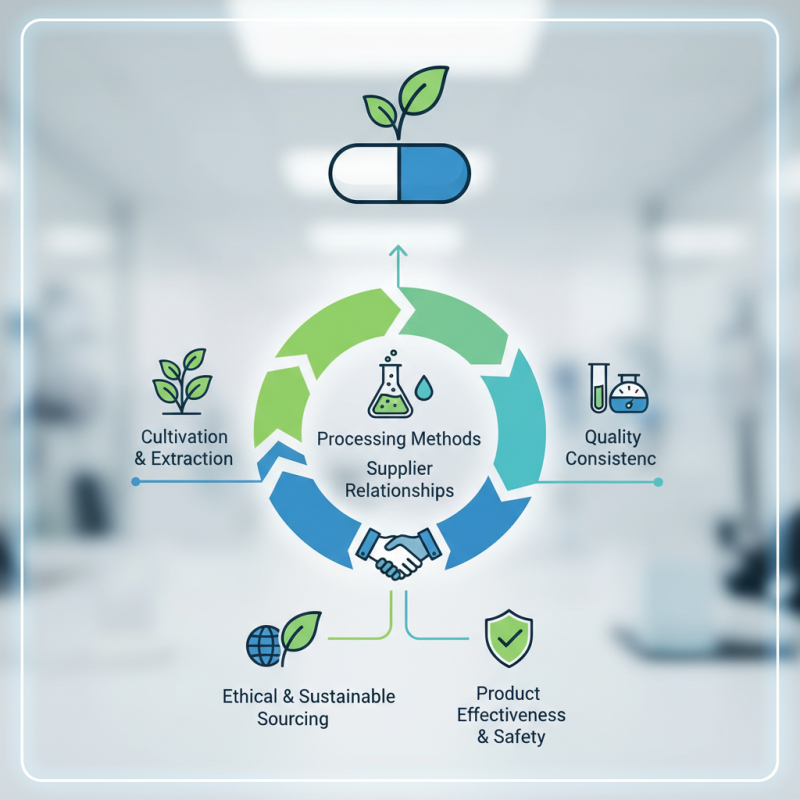
In the world of pharmaceuticals, the quality of Drug Raw Material is paramount. It directly affects the effectiveness and safety of medications. Sourcing these materials from reliable suppliers is crucial. Many factors influence the quality of these materials. For example, the cultivation, extraction, and processing methods can significantly impact the final product.
Quality consistency is often a challenge. Different suppliers may provide varying levels of purity. This inconsistency can lead to significant issues in production. It’s vital for manufacturers to establish strong relationships with raw material suppliers. Collaborating closely can ensure a steady supply of high-quality materials.
Furthermore, ethical sourcing of Drug Raw Material is a pressing concern. The environmental impact of sourcing practices should also be considered. Drug manufacturers must reflect on their sourcing choices. Balancing quality and sustainability can be difficult, yet it is essential for the future of pharmaceutical production.
Identifying Quality Drug Raw Material Sources in Pharmaceutical Production

Identifying quality drug raw material sources is crucial in pharmaceutical production. Quality materials ensure efficacy. They guarantee patient safety and consistency in products. Sourcing these materials requires a meticulous approach. Suppliers must be vetted carefully. Their production practices should meet rigorous standards.
Understanding geographical factors is important. Some regions may face challenges. Environmental issues can affect crop yields. This can lead to variable quality. By assessing these risks, producers can make informed choices. Regular audits of suppliers are necessary to maintain quality. Relying on third-party certifications may not always be sufficient.
Transparency in the supply chain remains a concern. Some suppliers may not disclose all sourcing details. This could lead to potential pitfalls. Building strong relationships with suppliers can foster trust. Communication is key to addressing issues as they arise. Producers should remain vigilant in their efforts. Quality raw materials create a strong foundation for effective drugs. However, the journey to secure these materials is ongoing and requires constant reflection.
Key Factors Affecting the Quality of Raw Pharmaceutical Materials
Quality raw pharmaceutical materials are crucial for effective drug production. Several key factors can significantly impact this quality. The source of these materials is fundamental. Sourcing from reputable suppliers guarantees integrity and efficacy. However, many overlook this aspect, leading to defective products.
The purity of raw materials is vital. Contaminants can originate from various stages: cultivation, harvesting, or storage. All these steps must be monitored to prevent impurities. Regular audits and testing help identify issues early on. Transparency in the supply chain is often lacking, resulting in hidden risks.
Tips: Always request Certificates of Analysis from suppliers. This document verifies material quality. Engage in open communication with suppliers. Build relationships to ensure consistent quality and reliability. Regularly review your sourcing strategy. Adjust based on feedback and market changes, as complacency can lead to significant quality lapses.
Regional Variations in Drug Raw Material Sourcing
Regional variations significantly influence drug raw material sourcing. Different regions have unique environmental conditions, local regulations, and resource availability that affect the quality of pharmaceutical production. For instance, some areas may have abundant natural resources, yet face challenges in refining them. Meanwhile, others might boast advanced technology but lack access to essential raw materials.
In Asia, many countries have established themselves as key suppliers. Their rich biodiversity offers various plant-based raw materials. However, quality control can be inconsistent. There are often variances in standards from one country to another. This can lead to concerns about the efficacy and safety of the materials sourced.
In Europe and North America, stricter regulations ensure higher quality products. Nevertheless, this can result in higher costs and longer lead times. Manufacturers must balance quality with affordability. Some may resort to less stringent sources to cut costs. This presents a dilemma—how to maintain quality while managing expenses in a competitive market.
Sustainability and Ethical Considerations in Raw Material Procurement
The pharmaceutical industry faces a significant challenge in sourcing raw materials sustainably. According to a report from the World Health Organization, over 80% of active pharmaceutical ingredients (APIs) are sourced from developing countries. This raises concerns about the environmental impact and labor practices in these regions. Many sourcing practices can lead to deforestation and biodiversity loss. There’s a growing need for transparent supply chains.
In a recent study by the United Nations, nearly 45% of pharmaceutical companies reported difficulty in ensuring ethical procurement. This includes concerns over fair wages and safe working conditions for laborers. Companies now emphasize eco-friendly sourcing methods. They aim to minimize their carbon footprint. However, implementing these practices can be complex and costly. Many organizations still rely on traditional methods, which can hinder progress.
Ethical considerations need focus. A balance between cost and sustainable practices is crucial. Some companies are exploring local sourcing to reduce transportation emissions. But not all manufacturers can adapt quickly. Challenges remain in enforcing ethical standards globally. Industries must continuously reflect on these practices and push for reform. They must prioritize both quality and sustainability as integral parts of their production process.
The Best Drug Raw Material Sources for Quality Pharmaceutical Production? - Sustainability and Ethical Considerations in Raw Material Procurement
| Raw Material Source | Sustainability Rating | Ethical Procurement Practices | Environmental Impact | Cost Efficiency |
|---|---|---|---|---|
| Plant-Derived Compounds | High | Certified organic sourcing | Low | Moderate |
| Minerals | Medium | Fair trade principles | Moderate | High |
| Animal-Derived Products | Low | Cruelty-free standards | High | Low |
| Synthetic Chemicals | Medium | Compliance with regulations | Low to Moderate | High |
| Biotechnology-Derived Products | High | Sustainable innovation practices | Low | Moderate |
Emerging Trends in Drug Raw Material Supply Chains and Quality Assurance
In recent years, the pharmaceutical industry has faced increasing challenges regarding drug raw material supply chains. According to a 2022 report by the World Health Organization, around 25% of global medicines are affected by raw material shortages. These shortages arise from geopolitical tensions, natural disasters, and regulatory issues. Manufacturers must frequently adapt to these disruptions to maintain production quality.
Quality assurance in the supply chain is crucial. Industry standards demand rigorous testing of raw materials. However, not all suppliers meet these standards. A survey by the Pharmaceutical Supply Chain Initiative revealed that 40% of companies reported quality issues with their raw material suppliers last year. This lack of consistency raises concerns about drug safety and efficacy.
Emerging trends suggest a shift towards localized sourcing. Many companies are revisiting their supply chains to reduce dependency on distant suppliers. This approach can enhance traceability and accountability. Despite this trend, challenges remain. Local suppliers may lack advanced quality controls. Companies must evaluate whether localized sourcing truly offers better quality or just convenience. As the industry evolves, commitment to quality must remain the primary focus.
Related Posts
-

5 Expert Tips to Source High-Quality Pharmaceutical Raw Materials in 2023
-

How to Optimize Your Supply Chain for Drug Raw Material Sourcing and Compliance
-
5 Essential Tips for Sourcing Quality Pharmaceutical Materials
-

Essential Tips for Sourcing Pharmaceutical Intermediates?
-

7 Best Strategies for Sourcing High-Quality Pharmaceutical Intermediates in 2023
-

Solutions for Sourcing the Best Pharma Materials in the Industry
