
2026 Best Pharmaceutical Testing Methods for Quality Assurance?
In the rapidly evolving pharmaceutical landscape, effective quality assurance is paramount. Pharmaceutical Testing plays a crucial role in ensuring that products meet strict safety and efficacy standards. Companies must adopt innovative testing methods to identify potential issues early in the development process.
It is essential to recognize the limitations of current methods. Some traditional practices may not detect all impurities or variations in drug composition. This can lead to unforeseen issues down the line. Physicians and patients rely on these products for health, making precision in testing non-negotiable.
As we head into 2026, exploring the best pharmaceutical testing methods is vital. These advancements will shape the future of quality assurance in the pharmaceutical industry. By embracing new technologies and techniques, we can ultimately enhance patient safety and trust in medical products.

2026 Trends in Pharmaceutical Testing for Quality Assurance

In 2026, pharmaceutical testing trends are evolving. The focus is shifting towards advanced automation and data analytics. These technologies improve efficiency and precision. They also pose challenges. Not all companies have adapted, leading to potential gaps in quality assurance.
Another emerging trend is the use of artificial intelligence. AI can analyze large data sets quickly. This helps identify anomalies faster than traditional methods. However, reliance on AI raises concerns about transparency. Decisions made by algorithms may lack human oversight.
Regulatory bodies are also updating guidelines. They emphasize the need for real-world evidence in testing. This approach aims to align drugs with patient outcomes. Yet, integrating this evidence into existing frameworks can be complex. Companies must ensure their processes remain robust while adapting to these changes.
Key Pharmaceutical Testing Methods: An Overview of 2026
Pharmaceutical testing methods are critical for ensuring drug quality. As we look toward 2026, several key methods emerge as essential. One of these is high-performance liquid chromatography (HPLC). This technique helps in accurately separating and quantifying compounds in a formulation. It ensures the active ingredients meet required specifications.
Another important method is mass spectrometry. This technique provides detailed information about the molecular composition of drugs. It can detect impurities at very low levels. However, the complexity of this method often leads to challenges. Staff training is vital here. Many labs struggle with the steep learning curve, leading to inconsistent results.
Microbiological testing is also crucial. It assesses the sterility of products and potential contamination. Employing proper techniques is necessary but challenging. Many facilities face limits in environmental control. This may lead to unexpected microbiological growth, raising concerns about product safety. Continuous improvement in these areas is essential for maintaining high standards in pharmaceutical testing.
2026 Best Pharmaceutical Testing Methods for Quality Assurance
| Testing Method | Purpose | Benefits | Limitations |
|---|---|---|---|
| High-Performance Liquid Chromatography (HPLC) | Separation and quantification of compounds | High precision and accuracy | Requires skilled personnel |
| Gas Chromatography (GC) | Analysis of volatile substances | Rapid analysis time | Limited to volatile compounds |
| Mass Spectrometry (MS) | Molecular weight determination and structure elucidation | Highly sensitive and specific | Expensive and complex instruments |
| Nuclear Magnetic Resonance (NMR) | Molecular structure analysis | Provides detailed structure information | Requires large amounts of sample |
| Dissolution Testing | Assessment of drug release from dosage forms | Predicts bioavailability | May not simulate in vivo conditions |
| Qualitative and Quantitative Microbial Testing | Microbial contamination assessment | Ensures product safety | Time-consuming |
Impact of Regulatory Changes on Pharmaceutical Quality Assurance Testing
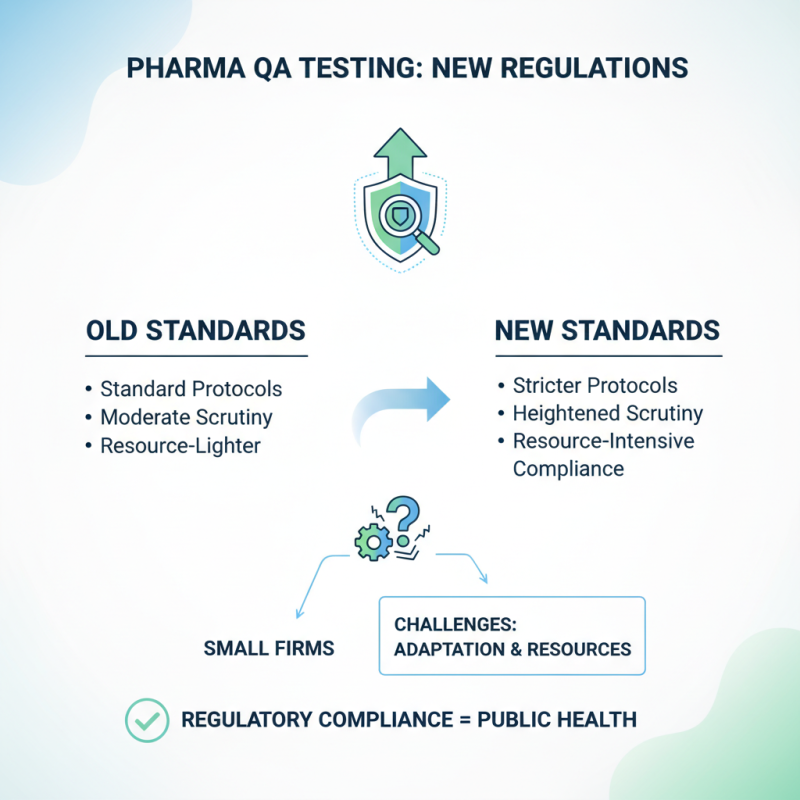
Recent regulatory changes have significantly influenced pharmaceutical quality assurance testing. These alterations demand more stringent testing protocols. Companies now face heightened scrutiny from regulatory bodies. Compliance can be overwhelming for some smaller firms. They often lack the resources to adapt swiftly to these new demands.
Incorporating advanced technologies into testing processes has become essential. However, not all companies are prepared to implement complex systems. Many still rely on outdated methods. This can lead to inconsistencies in product quality. The challenge lies in balancing compliance with innovation. Adapting to rapid changes can be daunting.
While the push for higher standards is beneficial, it can create unintended consequences. Some firms may rush to meet regulations, compromising product integrity. It's crucial for the industry to establish realistic timelines. Robust training programs can also help. Adequate education on regulations is necessary for all employees. Only then can companies ensure quality and safety without sacrificing innovation.
Emerging Technologies in Pharmaceutical Testing: 2026 Innovations
In 2026, the pharmaceutical testing landscape is set to undergo significant transformation. Emerging technologies are paving the way for enhanced quality assurance processes. Techniques like artificial intelligence and machine learning are becoming crucial. They can analyze vast datasets quickly, identifying anomalies in testing results.
One report indicates that AI can reduce testing time by up to 50%. This innovation will drastically improve efficiency. However, reliance on technology also raises concerns. Human oversight remains critical to avoid misinterpretation of AI-generated data.
Tip: Always maintain a balance between automated systems and human expertise. Regular training for personnel involved in testing can help address gaps that technology might overlook.
Another exciting development is the use of advanced biomarker analysis. This method helps in understanding drug efficacy with greater precision. But, this method can be complex. Variability in biological responses can lead to inconsistent results.
Tip: Implementing standard operating procedures (SOPs) is essential. SOPs can help navigate the uncertainties of new testing methodologies, ensuring consistent quality throughout the testing process.
Statistical Approaches in Quality Assurance for Pharmaceuticals in 2026
In 2026, pharmaceutical quality assurance relies heavily on statistical approaches. These methods enhance the reliability of testing processes, ensuring product safety and efficacy. A report from the International Society for Pharmaceutical Engineering indicates that 90% of pharmaceutical companies utilize advanced statistical tools for quality control. This trend reflects a growing recognition of quantifiable data in maintaining standards.
Statistical process control (SPC) is a primary method employed. It uses data to monitor and control processes, helping to identify variations. Notably, organizations using SPC have seen a 30% reduction in batch failures. However, challenges remain in data interpretation and the quality of underlying data. Many companies struggle with integration across different departments, leading to inconsistencies.
Moreover, predictive analytics is becoming essential in risk management. By analyzing historical data, firms can forecast potential quality issues. As per recent findings, 70% of companies report improved decision-making through these analytics. Yet, the reliance on technology raises questions about data security and accuracy. Companies must continually assess their methods to avoid over-reliance on flawed data.
Related Posts
-
Why Pharmaceutical Testing is Essential for Drug Safety and Efficacy
-

Understanding the Impact of Pharmaceutical Testing on Health Outcomes
-

Revolutionizing Drug Safety: The Future of Pharmaceutical Testing in Modern Medicine
-
The Ultimate Guide to Sourcing the Best Pharmaceutical Materials for Global Procurement
-

10 Best Practices for Peptide API Manufacturing to Maximize Efficiency
-

Ultimate Guide to Choosing the Right Pharmaceutical Supplier for Your Business
